About us
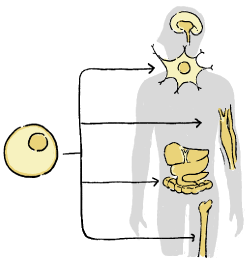
What is regenerative medicine?
Regenerative medicine is a medical treatment that aims to repair the functions of the body lost due to illness or injury using cells and various biomaterials. Unlike organ transplantation, cells used in regenerative medicine are processed by cell culture, gene transfer, or combined with materials that are safe and biocompatible.
Challenges in regenerative medicine
Regenerative medical products are processed materials that are used in the treatment of regenerative medicine.
There are two types of regenerative medical products that use cells: Autologous regenerative medical products that are made using the patient's own cells, and allogeneic regenerative medical products that are made using cells from a third party.
Currently, more than 10 regenerative medical products are approved in Japan, however, most of them are autologous products. On the other hand, there are only a few products made from allogeneic cell sources.
Autologous regenerative medical products have the advantage of minimal immunological rejection when administered, however, they also have the disadvantage of not being able to be used in an emergency because of the long time required for manufacturing the product.
In comparison, allogeneic regenerative medical products can be made in large quantities making inventory management possible, allowing more patients to benefit from these products when needed.
However, obtaining cells from a third party is difficult in Japan. Although efforts to establish a system to obtain and provide cells to pharmaceutical companies have begun, there are still many ethical and social issues to solve.
Efforts by Keio University Hospital for to provide a stable source of stem cell materials for regenerative therapy products
At Keio University Hospital, we are working on the " Project to accelerate the stable provision of stem cell materials in regenerative therapy products" with the aim of creating a system to make regenerative medicine available to more patients.
This project aims to activate the development of regenerative therapy products by providing a stable source of tissues and cells that are normally discarded during surgery to pharmaceutical companies that plan to manufacture and develop such products. The project is supported by the “Basic Technology Development Project for the Industrialization of Regenerative Medicine and Gene Therapy”, which is funded by the Japan Medical Research and Development Organization (AMED).
In the future, we will aim for a system that can supply a variety of cell raw materials in a more stable and sustainable manner with the cooperation of not only our hospital, but also other medical institutions.
